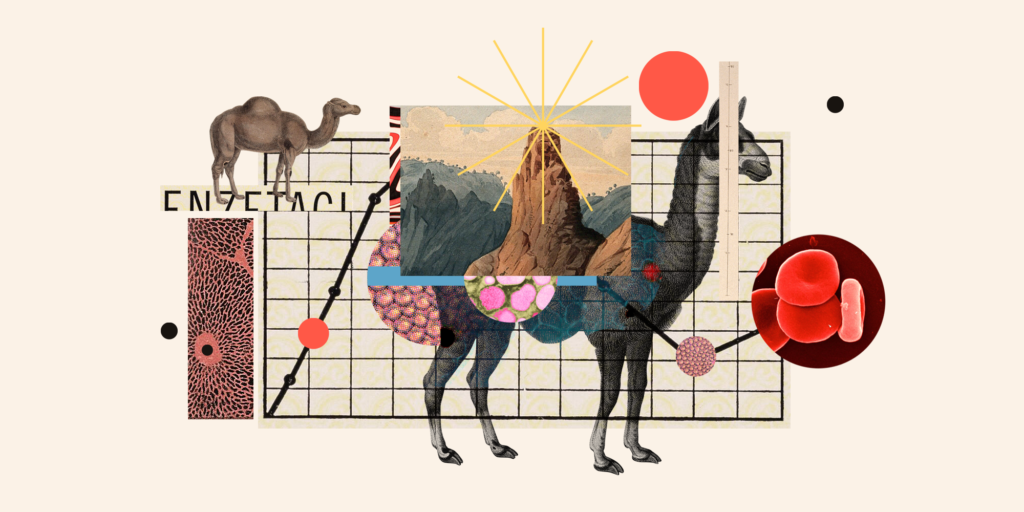
In the visualization that became ubiquitous in the first half of 2020, the coronavirus SARS-CoV-2 appears as a black ball covered in red floret “spikes”— proteins that allow the virus to stick to receptors at the surface of human cells. By all accounts, SARS-CoV-2 does this quite well. Our antibodies, composed of “heavy” and “light” chains that look like floating, primary-colored pasta piles, can hold off the virus by blocking the spikes from binding with these receptors, possibly stimulated by a vaccine. Late in May 2020, a team of American, Belgian, and German scientists reported on an antibody isolated from immunized animals that might neutralize coronaviruses in this way, meriting “further investigation as a potential therapeutic for the ongoing COVID-19 pandemic.” The surprising thing was the source of these antibodies: llamas.
At a moment when any cause for hope was cause for celebration, reporters jumped on the story. The New York Times anticipated llamas becoming “coronavirus heroes,” and just over a week later, The Guardian queried whether llamas “could be our secret weapon” against the disease. The New Yorker even pivoted to video to ask, “Can llamas save us from the coronavirus?” The reports were part of an early barrage of wishful thinking about laboratory animal research, each touting the potential for some new species to aid in coronavirus research — building public confidence and grant funding in equal measure. How did this humble camelid from the Andes become the source of so much hope?
Over the last three decades, llamas have quietly become a subject of tremendous fascination and investment in the world of speculative biotechnology. The possibility of a llama-based treatment for COVID-19 was, in fact, only the latest in a long string of attempts to position the animal as a key player in global scientific research and the profit margins of major pharmaceutical corporations. While the llama vaccine was ultimately beaten to the punch by less creaturely approaches, llama-based pharmaceuticals are almost certain to become an ever-larger part of the contemporary pharmacopeia in the years to come. According to consulting group G&L Scientific, “llama pharma” exemplifies the “innovation at the heart of the industry.”
This path forward might just as easily have been missed were it not for three decades of unexpected twists in the world of molecular biology, all occasioned by extra vials of camel blood in a Brussels freezer in the 1980s, and a discovery that molecular biologist Serge Muyldermans of the Vrije Universiteit Brussel described to me as “serendipity.”
Retracing that cold chain of events shows not only why llamas emerged as feasible COVID-19 saviors, but also reveals the emergence of a powerful formation in contemporary biotechnology; one we might call “llama biocapital.” Today, multiple drugs with multi-billion-dollar value are made from the stuff of llamas. This is part of a decades-long rush to develop so-called “nanobody” medicines that has provoked accusations of global biopiracy and potentially recast human immunology. If the most bullish scientific and corporate claims are to be believed, llama biocapital will redefine human health in the 21st century.
Studies in Cold Blood
The llama’s rise to biomedical prominence begins in 1981, when another pandemic was spreading fear around the globe: HIV/AIDS. In the United States, the CDC developed a short-hand for people they saw as high-risk groups: the so-called 4H club: Homosexuals, Haitians, heroin addicts, and hemophiliacs, with emphasis on the first. In France, daily newspaper Libération classified AIDS as an “Epidemic of Gay Cancer.” Yet over the Leie River in Belgium, where HIV reached popular awareness after a minor delay, the virus was figured very differently. Tabloids focused not so much on “homosexuals” as the source of the disease in Belgium, according to anthropologists Charlotte Pezeril and Dany Kanyeba, but “non-resident aliens,” particularly those traveling from the former colony in Zaire (now the Democratic Republic of the Congo). HIV was thus coded as “African” and “imported” when it was first publicly acknowledged in Belgium in 1983.
That explicit racialization of AIDS is, in history’s crystalline hindsight, predictable. While difficulties remain in ascertaining HIV’s exact origin, studies suggest the viral leap behind the epidemic occurred in the early decades of the 20th century along the banks of the snaking Congo River, near Kinshasa — then Léopoldville, capital of the Belgian Congo. Scholars have argued that the region’s busy railways and Léopoldville’s sex trade acted as central elements enabling the virus’s initial transmission. With the treatment and management of “tropical” diseases vital to the exercise of colonial power, the genocidal King Leopold II had opened the School voor Tropenziekten (today’s Institute of Tropical Medicine Antwerp) in 1906. Close connections and frequent travel, even after the DRC’s independence in 1960, meant that unfamiliar diseases routinely made their way overseas, back to Belgium, for study.
With AIDS cases surging locally in 1991, students at the Vrije Universiteit Brussel (VUB) refused to complete their final immunology exam. Expected to isolate antibodies from a sample of human blood and find molecular weights for the polypeptide chains therein, the students voiced fears about possible contamination. Serge Muyldermans, then a postdoc in the VUB lab assisting with the exam, recommended using mouse blood as a substitute, but the students resisted once more, arguing that sacrificing mice was counter to the mission of their studies—protecting life—and that murine blood work was amply documented in their textbooks.
The students’ professor, Raymond Hamers, was an unconventional academic: an unreformed hippy with a loose beard and unruly shock of hair who pursued problems, in history and literature as much as structural chemistry, with peripatetic zeal. One outgrowth of those roaming interests was a new postgraduate program in “Tropical Molecular Biology” (TMB), founded around 1982. The TMB program coincided with a turn of Hamers’s attention to livestock diseases in the Global South, and Hamers-trained students dispersing around the world, returning samples of animal blood back to VUB for further analysis. One student, Oumar Diall, sent Hamers extensive samples from local camels near his home in Mali, where the animals were economically significant for transportation as well as milk and meat, yet little-studied for their diseases.
Either Hamers or his wife, scientist Cécile Casterman-Hamers, decided that extras from Diall’s samples could be used for the students’ exam instead. Expecting little to come of their efforts except a temporary end to obstinance, camel blood was passed out for the exam. Yet the students returned with a surprising result: Within the samples were typical mammalian antibodies, with heavy and light chains, but also a strange, antibody-like material with a noticeably lower weight and no light chains. On first look, the curious results seemed to suggest one of two sources of failure: either the students had made a mistake, or the samples had deteriorated in transit from Mali, a routine problem when chilled blood traveled along the planet’s transnational cold chain. But a third possibility also presented itself, one that many in the lab initially refused to accept — perhaps there was something fundamentally novel in the blood of dromedaries.
To confirm the hypothesis, Hamers and his colleagues extracted blood from camels at the Antwerp Zoo. The antibody isolation process returned the same results: a lighter, fragmentary antibody. The students had apparently stumbled upon something unique to camels — a revelation that shook the foundations of knowledge not just about dromedaries, but immunology itself.
A Novel Antibody
The most common mammalian antibodies have a rough Y shape: the “arms” are variable, to match a bewildering array of invaders, while their “stems” are constant, so each antibody can connect with the rest of the immune system. This “Y” picture was first offered 30 years before the research in Brussels by early molecular immunologists Rodney Robert Porter and Gerald Edelman. In 1959, Porter cleaved antibodies in rabbit blood into fragments, which connect, he argued, in a linear “chain.” Around the same time, Edelman split the disulfide bonds within antibodies to reveal the existence of multiple chains. In 1961, these were subdivided into “light” and “heavy.”
Because both used rabbit blood as their experimental raw material, Porter and Edelman’s dominant vision of the antibody was one with two types of chains. The student discovery in Brussels, of a smaller antibody without Porter and Edelman’s “light” chains, thus ran counter to paradigmatic, Nobel Prize-winning findings. The prospect of a new kind of antibody, however, also came with a second possibility: Rather than extraneous elements of a deficient camel immune system, the smaller antibodies might have special utility in fighting invaders. Hamers and others wondered if they could induce an immune response in a camel to reveal how that process worked, but the Antwerp Zoo was less than thrilled about its limited “ships of the desert” becoming guinea pigs for routine experimentation with viruses like influenza. Another source of camels became urgently necessary.
The lab found one in Morocco. Drawing on an academic partnership in Morocco, the Hamers lab pooled money late in 1991 to purchase a camel that would be kept near a veterinary school in Rabat. The camel was immunized, and the lab awaited an opportunity to inspect its antibodies. Yet after a few months, when it came time to collect samples, news arrived that the camel had disappeared without a trace — a mystery still today. With limited funding, the team could hardly afford to purchase another. However, Nayib Bendahman, a technician in Hamers’ lab, had begun taking frequent trips to Morocco to see his father after the latter suffered a stroke. Bendahman, whose uncle owned a camel in the Atlas Mountains, was enlisted to slowly immunize the camel during his marathon motorcycle trips from Belgium to Morocco. More than a year later, samples of camel blood finally returned to Brussels and demonstrated that the little antibodies did, indeed, fight off pathogens with impressive power.
The Trouble with Camels
The first mention of heavy-chain antibodies in the scientific literature appeared in a letter to Nature in June 1993. The paper, drafted initially by Hamers and then edited by Muyldermans to include updated citations, described “considerable amounts” of antibody-like material “in the serum of the camel (Camelus dromedarius).” These smaller “heavy-chain” antibodies, as the letter referred to the mysterious material, were reported to be “a feature of all camelids,” and to bind extensively to antigens. Profiting from the discovery was on many minds. Hamers’ work through the TMB program had brought two patents, starting in 1989, along with minor earnings. Big money, however, was not in tropical disease, but the deeper coffers of the burgeoning global biotechnology sector.
Smaller, “engineered” antibodies had been at the forefront of work in the biotechnology sector ever since César Millstein and Georges J. F. Köhler introduced the “hybridoma” technology for mass-producing identical antibodies in 1975. Yet because the process used mouse proteins which produced dangerous immune reactions in human bodies, interest faded until the 1980s, when Greg Winter and colleagues at the Medical Research Council Laboratory (MRC) of Molecular Biology in Cambridge were able to produce rodent antibodies that could be safely used in humans. Readers of Hamers and his colleagues’ camel study in Nature therefore recognized the value of small, naturally occurring antibodies. In 1994, a review of antibody design in pharmaceuticals cited the Brussels scientists’ findings prominently, and researchers at the MRC described efforts to “camelise” human genes shortly thereafter. In addition, and unbeknownst to many involved, the University of Miami’s Andrew S. Greenberg and others were actively engaged in work on a “new antigen receptor” (NAR) in nurse sharks shown to function similarly to the heavy-chain antibodies of camelids.
Fearing that their findings would be taken up by others, Hamers and his research partner/wife, Hamers-Casterman, paid nearly 25,000 euros of their own money to apply for a European patent on heavy-chain antibodies in August 1992, roughly a year prior to publication in Nature, and filed an international application the next year. The patents were sweeping in scope, but followed a path laid out by the June 1980 U.S. Supreme Court decision in Diamond v. Chakrabarty, which held that live microorganisms could be patented, and the 1988 U.S. Patent and Trademarks Office’s move to grant Harvard rights to “Oncomouse,” a creature genetically engineered for increased susceptibility to cancer. Ownership of the camel patents, however, was only one side of the equation. Incoming investment offers were something else entirely, and they were slow to arrive.
One person did see potential for the research: a representative from the Dutch conglomerate Unilever. A massive and unwieldy corporate entity that began dramatic restructuring in 1980, Unilever was focused on expanding its personal products division, which included shampoos, toothpastes, and detergents. Cornelis “Theo” Verrips, a Dutch molecular biologist who helped develop the Clearblue pregnancy test, had been tasked with using antibodies in a consumer toothpaste that would attack oral bacteria without producing antibiotic resistance. But after many attempts, a commercially viable product appeared impossible. As a last-ditch effort, Verrips convinced Unilever to give him space to research other antibody possibilities, including camel antibodies.
Eager to find collaborators interested in their findings, the VUB lab sent samples to Unilever. Yet when Hamers and his colleague Lode Wyns traveled to Unilever HQ, Wyns recalled their surprise at finding that its scientists had taken out a patent on the process, hoping to block the original camel research team at VUB from further research. The groups eventually settled their differences, and the result was a broad patent, filed by Unilever in 1994, that included Hamers, Hamers-Casterman, and Muyldermans, as inventors, which allowed the company to profit from future discoveries. However, despite the wide-ranging nature of the Hamers patents, Unilever found itself incapable of making blockbusters of the antibodies. The company developed an antibody-based detergent, but the powder turned brown in storage, which killed its commercial prospects. In October 1998, after an adulatory article on future llama-based products appeared in the Royal Dutch Chemical Association’s Chemisch Weekblad, a reporter for Dutch daily de Volkskrant found that Unilever had no such products in the pipeline.
Bring in the Llamas
The failure of Unilever’s detergent signaled a subtle shift in antibody research. In the years to come, camels fell away entirely, leaving llamas as the public face of “nanobodies.” The reasons had less to do with significant serological differences between the animals than some relatively basic facts of animal husbandry.
From the earliest days, camels had been a logistical challenge for scientists in the Brussels lab. Following his postdoc, Muyldermans temporarily solved the problem in the mid-1990s, after researchers at the Central Veterinary Research Laboratory (CVRL) in Dubai requested a reprint of a VUB paper and invited the professor to see their facilities. During the visit, the CVRL scientists agreed to immunize camels for free and send samples to Belgium in exchange for authorship on a paper emerging from the research. Thus a publication on enzyme inhibition by camel antibodies in 1998 features two researchers at the CVRL, and a paper from the next year by Muyldermans and Marc Lauwereys acknowledges “His Highness General Sheikh Mohammed Bin Rashid Al Maktoum for the financial support.”
The downside to these unexpected collaborations was obvious: preparing fresh materials in the laboratory and immediately injecting them into camels was logistically impossible. If the only camels close to VUB were at the zoo and inaccessible, a more proximate animal had to be found. The “New World” camelids — llamas or alpacas — represented the next logical choice. Early findings had shown that both species produced heavy-chain antibodies, and, despite a more limited cultural prominence than today, small farms had begun to raise llamas and alpacas for their wool in agricultural regions of Belgium. By 1997, VUB was focusing entirely on llamas. They were “easier to immunize, to breed, to raise,” and acclimated to colder temperatures over millennia in the Andes — therefore easier for scientists to imagine keeping in Northern Europe. Relatively few were needed for research purposes, because each produced more than enough post-immunization antibodies, so maintaining a small herd presented no real challenge. In fact, Muyldermans still has a few knocking around outside of his daughter’s house.
The animals were now alienated from their traditional homelands, truly at home only in the worldless world of patent law.
With llamas taking center stage, the VUB team rapidly published a series of additional studies on the antibodies just before the dawn of the new millennium, including findings that the antibodies inhibited enzymes, which signaled their potential for chemotherapy treatments or antibiotics. Their success was supported by an infusion of Flemish government funding into the molecular biology program at VUB, which lay in a propitious place for such work.
The U.S. Food and Drug Administration’s 1986 approval of Orthoclone OKT3, an immunosuppressant used to limit rejection in organ transplant patients, inaugurated the beginning of what many considered an “antibody revolution” in medicine, clearing a path toward substantial profit. Although progress was slow, eight other antibody-based drugs reached the market by 2001, and many more were in the pipeline, with broad possible applications from arthritis (Abbott Laboratories’ Humira would be approved in 2002) to cancer (Merck’s blockbuster Keytruda appeared in 2014). Because llama antibodies were smaller than conventional antibodies, they suggested the possibility of counteracting an even wider array of molecular invaders, possibly reaching and binding with viral and bacterial molecules that larger antibodies could not. Llama drugs represented a potential second phase of the antibodies revolution, and the Brussels researchers were one of the first teams selected to work with VIB, motivated by this promise and a sense that they were close to forming a lucrative startup.
Few on the VUB side had meaningful business experience. Jan Steyaert, a former member of Wyns’ Structure Biology lab, was brought in to develop a business plan and begin to pitch investors for their new company “MatchX” — so named because they were “matching” RNA pairs and every startup at that time seemed to need an “X” in its name. MatchX was incorporated in July 2001, though it changed its name a year later to “Ablynx,” a reference to the company’s work linking antibodies, the inevitable X, and a reach for alphabetic stock prominence. Its inaugural CEO, and “trump card” according to an early profile, Mark Vaeck, received a doctorate from the Hamers lab in 1982, just prior to the antibody discoveries, before climbing the ladder at a series of successful startups: Plant Genetic Systems (PGS), an early Belgian agro-biotechnology company that pioneered insect-resistant tobacco plants; pharma giant UCB, the company behind Zyrtec; and then Cetus, Keygene, and Ceres, Inc. In 2002, Vaeck brought this experience home to take the mantle at Ablynx, which soon raised 5 million euros in early investment.
With 40 patents and patent applications to its name based on the llama work, the young startup planned to grow quickly, producing returns on its intellectual property and increasing staff from 10 to 50 within two years. Vaeck decided early on that Ablynx would focus exclusively on developing therapeutics based on its proprietary llama “mini-antibody” products, joining a market for antibody-based medicines that would grow from 4 to 14 billion dollars in sales by 2005. Because the Hamers patents, now owned by Ablynx, covered all camelid “products,” the company could fend off competitors, with one key exception: the “Winter II” patent family (named for Greg Winter) protected creation of an antibody library, so Ablynx had to develop its first libraries in Portugal, where Winter II was not in force.
By 2006, with about twenty research llamas, Ablynx had dropped the “mini-antibody” nomenclature for a new term: “nanobodies.” A reference to “nanometer-sized antibodies,” the nano addition was motivated less by scientific concerns than marketing ones. Ablynx had struggled to explain “mini-antibodies” to investors and grant agencies as it geared up for trials on its first major drug candidate, caplacizumab. Now, instead, the company made “nanobodies,” which was sexier — if not ultimately much clearer to a layperson.
Peddling nanobodies, Ablynx succeeded in meeting the rapid growth goals envisioned by CEO Vaeck. “If there was such a thing as a popularity contest for small European biotechnology companies, Ablynx would be in with a good chance of getting a prize,” pharma trade publication EP Vantage wrote in 2008. That popularity contest became an acquisition war in 2018, when news spread that Ablynx was close to bringing caplacizumab to market. Entering clinical trials in December 2008 and receiving “Orphan Drug” designation the next year, caplacizumab was intended to treat a rare blood disorder known as acquired thrombotic thrombocytopenic purpura, which causes blood clotting. As the drug headed for approval in the European Union in 2018, Ablynx fought off acquisition by Danish pharmaceutical multinational Novo Nordisk for $3.1 billion dollars before agreeing to a second purchase offer from French pharma giant Sanofi for the gobsmacking sum of around $4.8 billion. With projected annual sales of only $500 million, caplacizumab was a treatment with a narrow clinical niche, but it was also a litmus test for antibody research in decades to come. Novo Nordisk and Sanofi weren’t merely betting on a drug, but the future of llama biocapital.
Who Profits from Llama Biocapital?
Historian and sociologist Hannah Landecker has argued that one of the most significant transformations in 20th century biology was the invention of cell cultures. Culturing made the body and its cells alienable, both conceptually and practically. The discovery of “heavy-chain antibodies” or “nanobodies” built upon this history: Rather than an innate characteristic of camels, the antibodies could be viewed and acted upon as distinct biological fragments. Patents meant they could also be owned and sold as “biocapital,” pieces of living matter subject to the whims of international markets. When the first Hamers patent with Unilever covered worldwide production of antibodies “derived from heavy chain immunoglobulins of Camelidae,” it effectively granted a Belgian company ownership over antibodies from any camel, llama, alpaca, or vicuña in the world. Because the vast majority of camelids were native to and lived outside of Europe, Low Country molecular biologists were making wealth from the blood of a vast number of animals with diverse biological and cultural histories. Such animals were now alienated from their traditional homelands as well, truly at home only in the worldless world of patent law.
That conclusion was far from satisfying to many around the world who claimed llamas as their own. As Ablynx engaged in negotiations over a possible acquisition in 2006, Peruvian journalist Ricardo Léon responded to BBC reporting on nanobodies by wondering whether they might constitute an example of “biopiracy.”
“The problem for Peru,” wrote Léon, “begins when it is discovered that the patent in question protects the basic structure, the composition, preparation, and use of the Nanobodies, but ignores the interests of the countries from which these species originate.” The Sociedad Peruana de Derecho Ambiental, an environmental nonprofit based in San Isidro, Peru, began investigating whether a case could be brought against Ablynx and other companies to force them to share their findings so that Peruvians would not pay full price for vaccines and diagnostics forged from their own bio-heritage. It would hardly be the first time that Peru suffered in this way: American entrepreneur Loren Miller notoriously won a patent for Banisteriopsis caapi, the vine used for the ceremonial, psychoactive beverage ayahuasca, hoping to develop treatments from it — although his claim was revoked in 2004.
Yet the fight against llama biopiracy vanished almost as quickly as it began. One problem for any Peruvian claim was the multiple alienation of camels, llamas, and HCAbs: of animal from place, antibody from organism, commodity from biomaterial. That camelids generally, not llamas or alpacas specifically, produce the useful fragments added a further wrinkle, evidence of the complicated intersections of evolutionary history and transnational capital. Critical accounts of Western bioprospecting in the Global South often emphasize incongruities between legal contracts and the structure of Indigenous knowledge, but what country or people could lay original claim to the evolutionary event, millions of years prior to domestication itself, that sent “heavy-chain antibodies” on diverse geospatial trajectories?
For scholar Hannah Landecker, “biotechnology changes what it is to be biological.” The advent of cryopreservation technologies, for instance, allowed life to be “paused,” frozen. The discovery and alienation of camelid antibodies also quietly changed human biology. Should heavy-chain antibodies really target molecular invaders more effectively than traditional antibodies, their nonexistence in humans could be interpreted not as something spectacular about camels, but as our deficiency. Muyldermans, Wyns, and French molecular biologist Christian Cambillau described this as “the superfluous luxury” of normal antibodies in 2001. Ten years later, Muyldermans and other collaborators reflected upon the longer evolution of HCAbs, noting “they are clearly useful and, anthropomorphically speaking, all species would love to have them.” Such a sentiment would have been nearly unthinkable only a few decades before, when antibodies were not yet so separable from the animal bodies that produced them. But as newly commodified biomaterial, members of the human species could soon line up to purchase them. Figured simultaneously as biocommodities and universal public goods, since “all species would love to have them,” camelid antibodies are trailed by entangled (post)colonial pasts and presents. Sanofi, for its part, makes an effort to signal good corporate “citizenship” by highlighting financial contributions to the fight against biopiracy in its annual reports.
The Wooly Path
Llama biocapital’s profits have not remained in Belgium. Although Muyldermans and Hamers, the scientists who helped launch nanobody-based research, purchased shares at Ablynx’s initial public offering, neither profited greatly from the company. The former reinvested much of what he earned into his own department, the latter endowed a chair in the History and Philosophy of Science at VUB. At the time of Sanofi’s acquisition of Ablynx, only about 16 percent of the company was in Belgian hands, with most of the profits heading to American investors — a dramatic disappointment for early hopes of keeping value in Brussels. “Everyone thinks that we sit on mountains of money because we founded Ablynx,” noted Steyaert in 2017, “but that is not consistent with the reality.” Indeed, the company’s tale remains a cautionary one about the failure of academic researchers to benefit from their findings once taken up into the engines of pharmaceutical biocapitalism.
Hamers retired in the early 2000s, but for Muyldermans, who was promoted to full professor at VUB in 2003 and did not join Ablynx, the commodification of nanobodies came with academic costs. Industrial partners turned to Ablynx for collaborations, not the VUB molecular biology department. Given the pharmaceutical industry’s tendency to prioritize chronic conditions with large markets, the researchers who did continue to work with VUB tended to focus on the areas where money was scarcer, particularly neglected, tropical diseases, which launched the camelid discoveries in the first place. Steyaert later rued that Ablynx’s early plans failed to protect academic collaboration and maintain VUB’s rights to unused intellectual property.
As VUB’s and later Ablynx’s research became increasingly entwined with the appreciable strangeness of the llama-as-test-subject issue, the origins of the camel blood frozen in VUB freezers was reduced to a humorous anecdote demonstrating the serendipity of the discovery for journalists, scientists, and the occasional historian. The postcolonial economic and political relationships, the South-North circulation of students, the focus on tropical diseases, and student fears of infection from an “African” disease — all became mere narrative set pieces for the incredible odyssey of llama biocapital. “Llama nanobodies” replaced “camel antibodies,” seemingly shorn of history.
In turn, the success of Ablynx and the early discoveries at VUB have today metamorphosed into a broader fascination with the multiple utility of llama antibodies. Belgian company argenx, formed in 2014 by former executives of Ablynx, also focuses on llamas, yet emphasizes their “conventional” antibodies, which are still smaller than typical mammalian antibodies and functional in binding to pathogens. Early in 2021, the company was one of the highest performing biotech stocks on the market, and a windfall is expected if the FDA approves its first drug candidate, efgartigimod, for treatment of Generalized Myasthenia Gravis, a chronic autoimmune condition with a patient population comparable to that targeted by caplacizumab. Belgian business magazine Trends wondered this February whether “argenx will finally transform the reputation of Belgian biotechnology into a veritable success story.”
A number of lower-profile llama advances have followed Ablynx, too. The recent lapsing of the Hamers patents means work with camelid antibodies, now often reproduced with genetically modified yeast, is widespread. Before llamas became potential coronavirus heroes, a number of studies explored their capacity for detecting infectious diseases. A 2010 report pondered the enlistment of llamas as “Soldiers in the War on Terror” by using the antibodies to identify bioweapons. Interest in using the antibodies for treatments has also been complemented by attention to their potential as biomarkers or biological tracers. By binding to specific molecular targets better than conventional antibodies and staying in blood circulation for shorter periods, nanobody tracers offer a path to “noninvasive detection” of cancer and other conditions. Llama antibodies have also been used to crystallize unstable proteins, including in work on G-protein-coupled receptors that won the 2012 Nobel Prize in Chemistry. At each turn and with every press release, patents followed, and llama antibodies surfed the promissory cycles of hype and hope that characterize the contemporary pharmaceutical industry.
In the last decade, researchers have shown that nanobodies might, in a minor historical irony, serve as a possible new treatment for HIV or aid in resisting the flu. The latter finding, combined with the awareness that camels act as reservoirs for diseases such as MERS, helps to explain the strange prominence of the llama in the fight against SARS-CoV-2. Forever ago in November 2018, Melissa Healy reported for the Los Angeles Times that new antibody research might reveal a pathway to the “universal vaccine against influenza” running through llamas. The National Institute of Health’s Anthony Fauci, before his celebrity turn in White House press briefings, “offered a full-throated appreciation for the new study.” Yet that vaccine, like much else promised of llama biocapital, remains somewhere in the future. As a 2018 report in PBS Nova punned, “The trek from llama to lab was long… and the path ahead may be just as woolly.”
The cycle nevertheless continues: In December 2020, the National Institutes of Health announced promising antibodies against COVID-19, isolated from a llama named “Cormac” — the first tentative but meaningful update on the camelid hopes of early 2020. One month later, a multi-country research collaboration shared word, in Science, of a treatment candidate based on llama nanobodies. ExeVir, a new startup spawned in 2020 out of VIB with plans to use nanobodies against COVID, raised $50 million in Series A funds in March. The company began Phase 1 clinical trials in human patients this August. Yet none of the Pfizer, Moderna, or Johnson and Johnson vaccines were built from the blood of camelids. The brighter future, one in which llama antibodies become the raw material for vaccines and treatments that defeat everything from the common cold to the world’s great pandemics, remains, for the moment, just out of reach. Whether its moment comes soon or after many decades of waiting, the promise and promises of llama biocapital show little sign of diminishing.